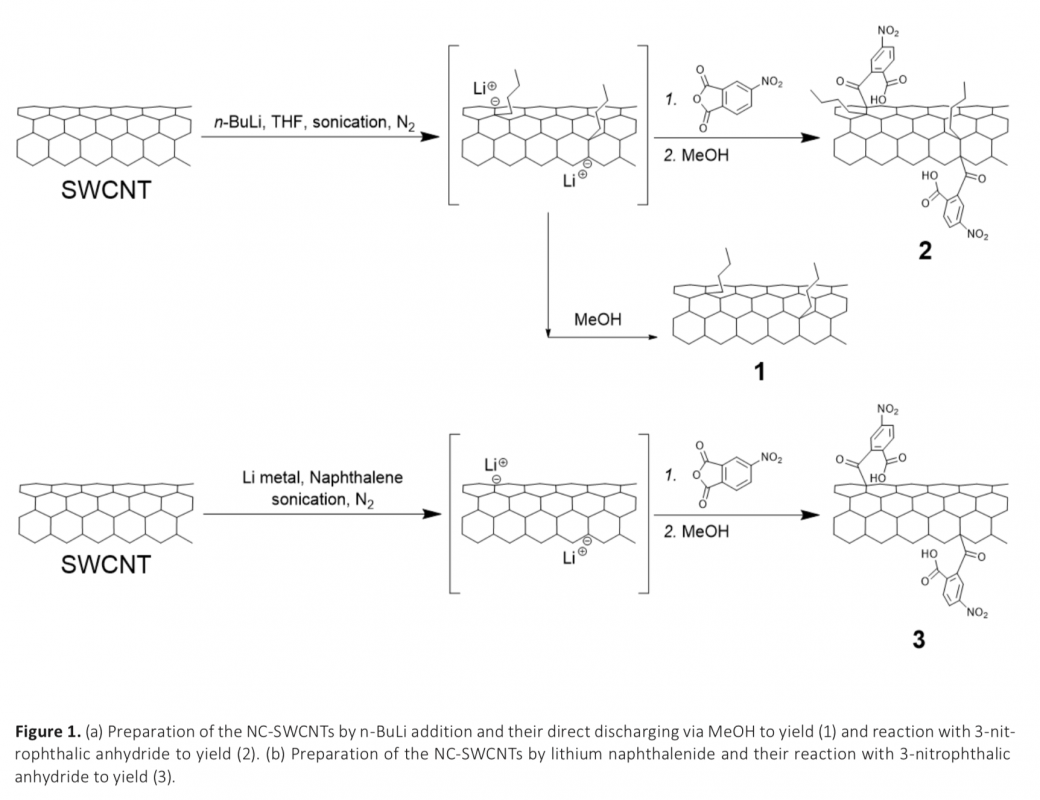
Reductive chemistries have widely been used to functionalize single-walled carbon nanotubes (SWCNTs). However, the reactivity of negatively charged SWCNTs (NC-SWCNTs), prepared by different reductive chemistries, to the same electrophilic reagent has not been evaluated. Here in, the first example of the reactivity comparison of two different NC-SWCNTs towards 3-nitrophthalic anhydride is presented, and two novel functionalized SWCNTs are synthesized and characterized. The NC-SWCNTs, that are denoted as [(nBu―SWCNTn)-•Lin+] and [SWCNTn-•Lin+], are prepared via n-butyl lithium and lithium naphthalenide addition, respectively, and are reacted by 3-nitrophthalic anhydride under dry conditions. The resulting functionalized SWCNTs are characterized by Raman, UV-vis-NIR, TGA-MS, XPS, and TEM. The reactivity of [(nBu―SWCNTn)-•Lin+] towards electrophilic 3-nitrophthalic anhydride is found to be higher than [SWCNTn-•Lin+]. This isprobably due to the high nucleophilic character of [(nBu―SWCNTn)-•Lin+] which bears lone pair electrons and electron- donating butyl groups.
İndirgenme kimyası tek duvarlı karbon nanotüpleri (TDKNT’ler) işlevselleştirmek için yaygın olarak kullanılmaktadır. Bugüne kadar, farklı indirgeyici kimyasallar tarafından hazırlanan negatif yüklü TDKNT’lerin (NY-TDKNT) elektrofilik reaktiflere karşı reaktivitesi değerlendirilmemiştir. Burada, iki farklı NY-TDKNT’nin 3-nitroftalik anhidrid’e karşı reaktivite karşılaştırmasının ilk örneği sunulmuştur ve iki yeni işlevselleştirilmiş TDKNT sentezlenmiş ve karakterize edilmiştir. Sırasıyla n-bütil lityum ve lityum naftalinit ilavesiyle hazırlanmış olan NY-TDKNT’ler ([(nBu― TDKNTn)-•Lin+] ve [TDKNTn-•Lin+]) kuru koşullar altında 3-nitroftalik anhidrit ile reaksiyona tabi tutulmuşlardır. Elde edilen işlevselleştirilmiş TDKNT’ler Raman, UV-vis-NIR, TGA- MS ve TEM ile karakterize edilmiştir. [(nBu― TDKNTn)-•Lin+]’nin 3-nitroftalik anhidrit elektrofile karşı reaktivitesinin [(nBu― TDKNTn)-•Lin+]’den daha yüksek olduğu bulunmuştur. Bu muhtemelen yalnız bir elektron çifti ve elektron veren bütil grupları taşıyan [(nBu― TDKNTn)-•Lin+]’nin yüksek nükleofilik karakterinden kaynaklanmaktadır.
Download Article in PDF (1.1 MB)