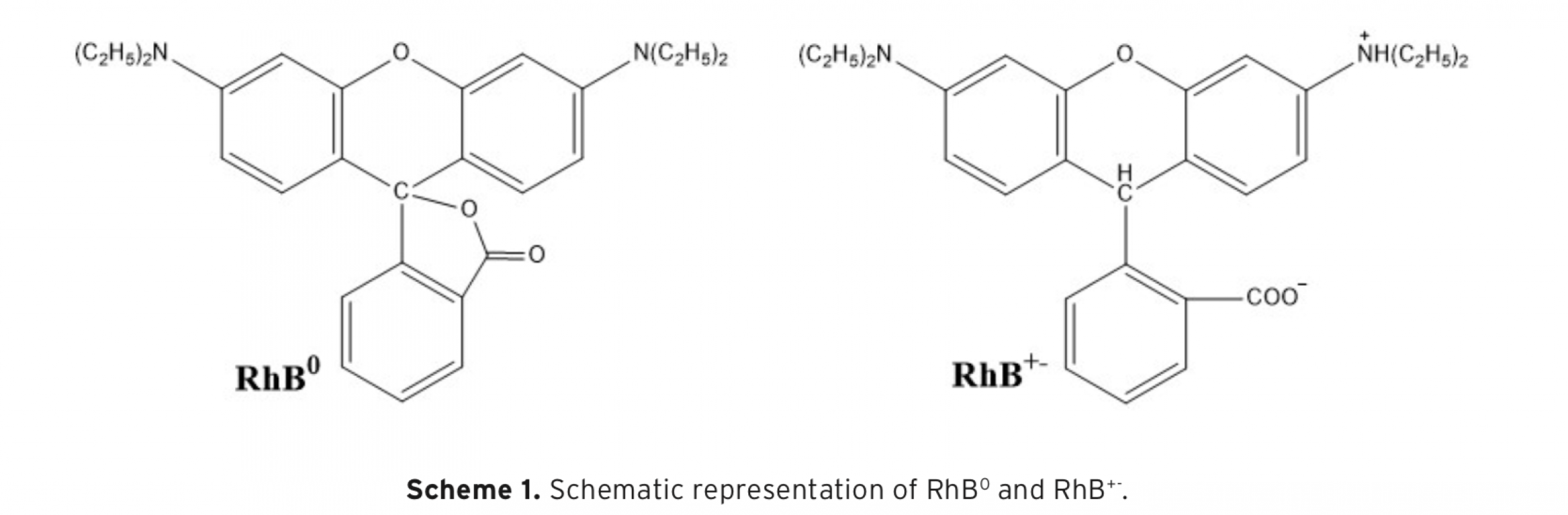
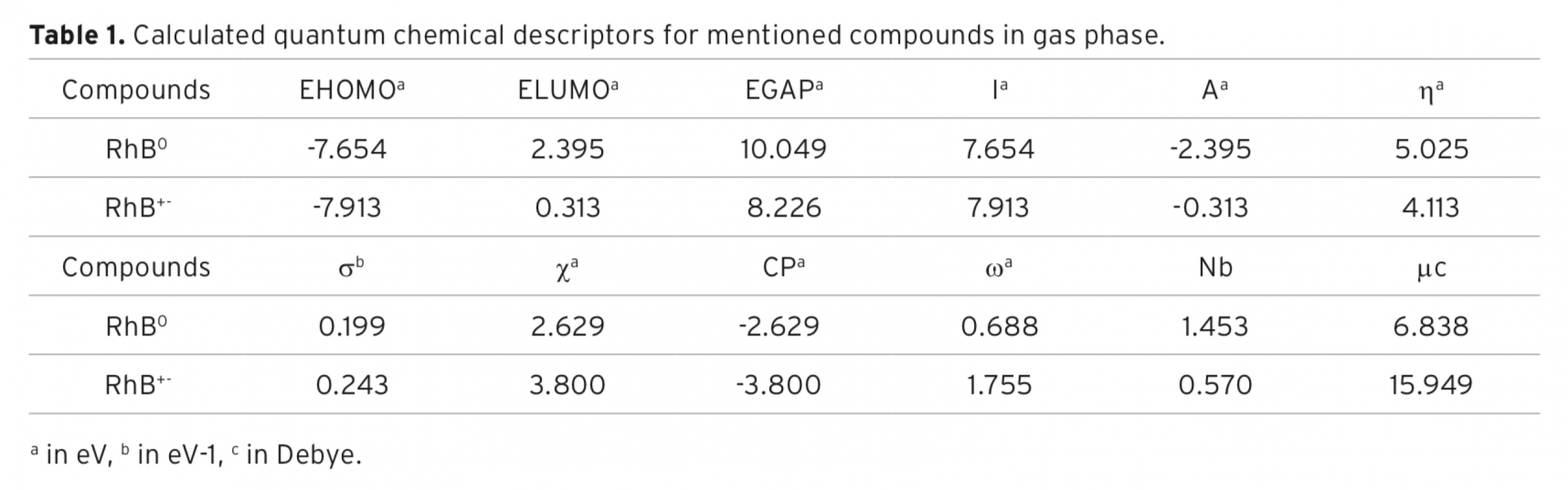
The aim of the study was comparatively to investigate decolourization of Rhodamine B (RhB) in aqueous solution using photocatalytic and ultrasonic processes. Also, computational investigations of RhB0 and RhB+- compounds were performed at HF/6-31G level in gas phase. Photocatalytic decolourization of RhB was studied using TiO2 and silver-loaded TiO2 (Ag-TiO2) as catalyst. It was found that decolourization by photocatalytic process of RhB increased with decreasing pH, and decolourization rate also increased in the presence of TiO2/ UV when compared to UV irradiation alone. Moreover, Ag-loading to TiO2 dramatically reduced decolourization time. The decolourization by ultrasonic process of RhB was also studied by using various salts and initial dye concentrations at various pHs and amplitudes. The decolourization by ultrasonic process of RhB was found to increase with decreasing pH, increasing amplitudes and addition of various salts to aqueous solution. It was observed that the decolourization decreased with increasing initial RhB concentration. The decolourization rate of the dye was monitored spectrophotometrically at 554 nm.
Bu çalışmanın amacı fotokatalitik ve ultrasonik yöntem ile sulu çözeltideki Rodamin B (RhB)’nin renk gideriminin karşılaştırmalı olarak araştırılmasıdır. Ayrıca, RhB0 and RhB+- nin gaz fazında HF/6-31G seviye- sindeki hesaplamalı araştırılması da yapılmıştır. RhB’nin fotokatalitik renk gideriminde TiO2 ve gümüş yüklü TiO2 (Ag-TiO2) katalit olarak kullanılmıştır. RhB nin fotokatalitik yöntemle renk gideriminin azalan pH ile arttığı, ayrıca renk giderim oranının sadece UV ışığı ile ışınlamayla kıyaslandığında TiO2/UV varlığında daha da arttığı gözlenmiştir. Ag-yüklü TiO2 in kullanılması ise renk giderme zamanını önemli ölçüde azaltmıştır. RhB nin renk gideriminde çeşitli tuzların, farklı pH’larda boya başlangıç derişiminin ve ses dalgası şiddetinin etkisinin de araştırıldığı ultrasonik yöntemle de çalışılmıştır. RhB nin ultrasonik yöntemle renk gideriminin azalan pH, artan ses şiddeti ve sulu çözeltiye eklenilen çeşitli tuzların etkisiyle arttığı gözlenmiştir. Renk gideriminin RhB başlangıç derişiminin artmasıyla azaldığı gözlenmiştir. Boyanın renk giderim oranı 554 nm’de spektrofotometrik olarak izlenmiştir.

Download Article in PDF (557.0 kB)