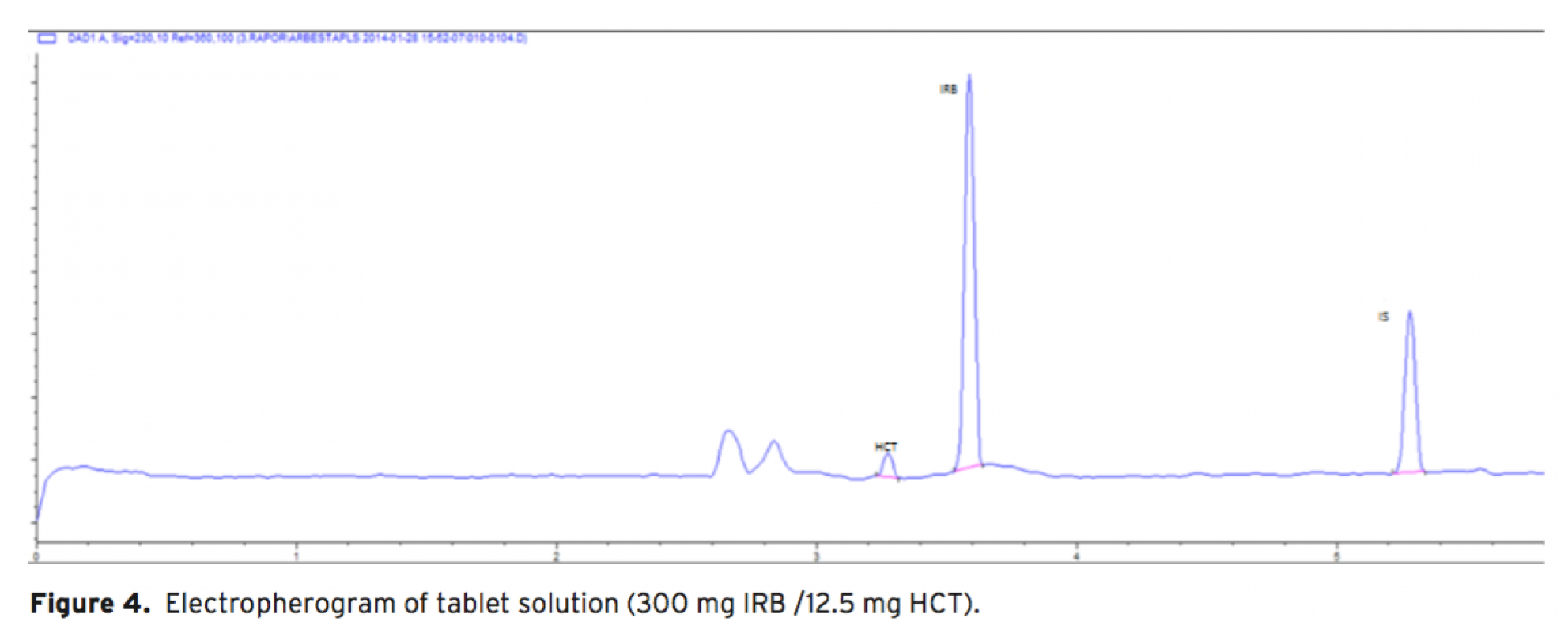
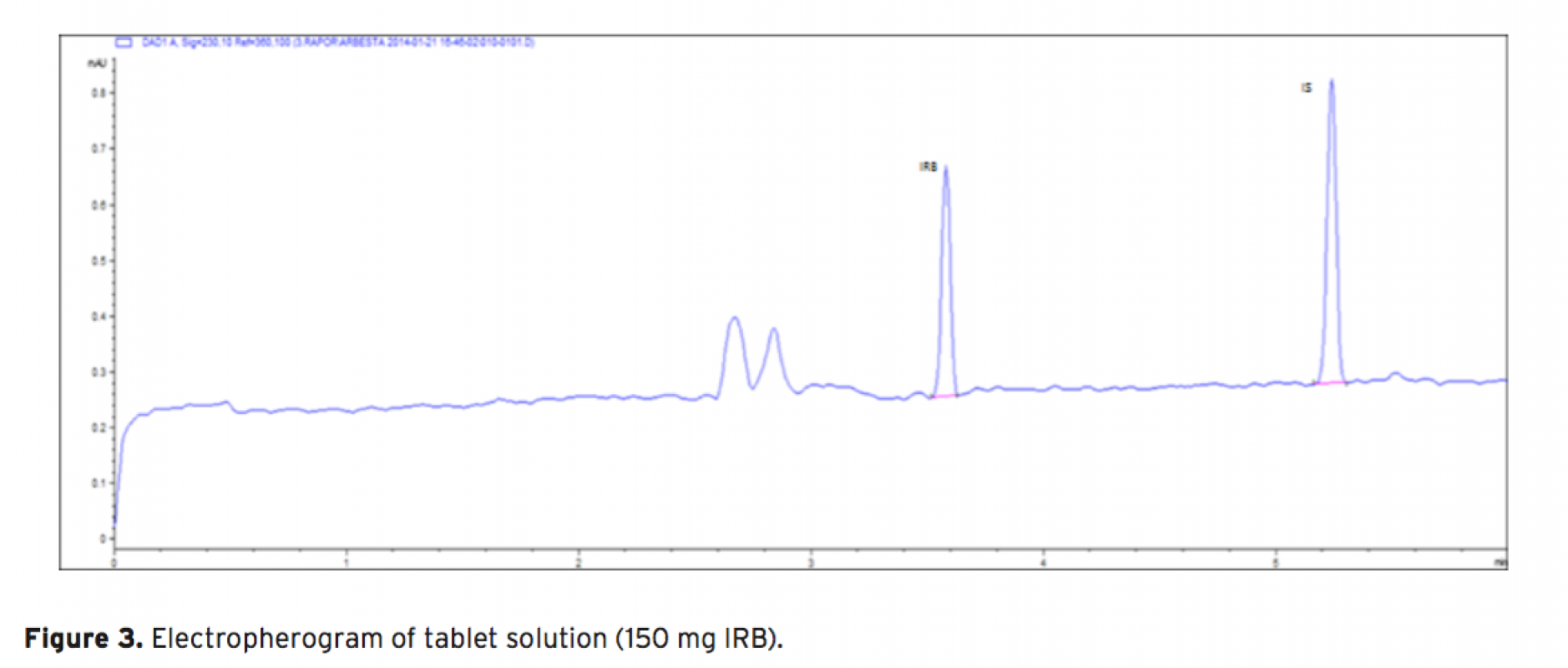
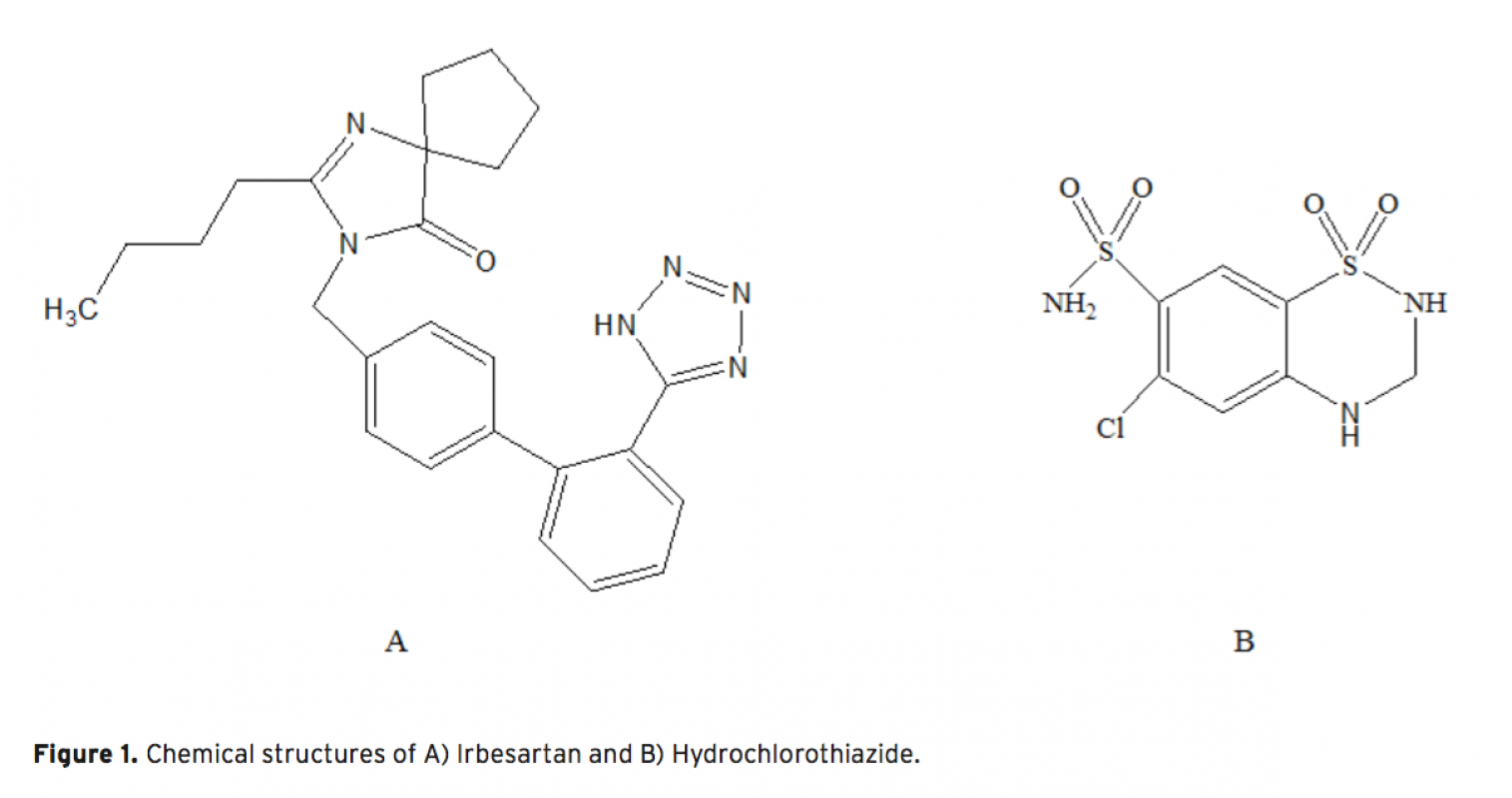
Acapillary electrophoretic method was developed and validated for the simultaneous determination of irbesartan and hydrochlorothiazide in tablets. The run buffer was used 10 mM borate buffer containing 10% methanol (pH 9.0). Compounds were separated less than 6 minutes. The limit of quantification (LOQ) values were 3.370×10-7 M (0.144 μg/mL) and 3.140×10-7 M (0.094 μg/mL) for irbesartan and hydrochlorothiazide, respectively. The method was applied successfully to both irbesartan and irbesartan/hydrochlorothiazide combi- ned tablets with good recoveries almost 100%.
İrbesartan ve hidroklorotiazidin tabletlerde eş zamanlı analizi için bir elektroforetik yöntem geliştirilmiştir. %10 metanol içeren 10 mM borat (pH 9.0) çalışma tamponu olarak kullanılmıştır. Bileşikler 6 dakikadan daha düşük sürede ayrılmıştır. Tayin sınırı (LOQ) değerleri irbesartan için 3.370×10-7 M (0.144 μg/mL) ve hidroklorotiazid için 3.140×10-7 M (0.094 μg/mL)’dir. Yöntem, irbesartan ve irbesartan/hidroklorotiazidi birlikte içeren tabletlere yaklaşık %100 gibi bir geri kazanımla başarılı bir şekilde uygulanmıştır.

Download Article in PDF (210.2 kB)