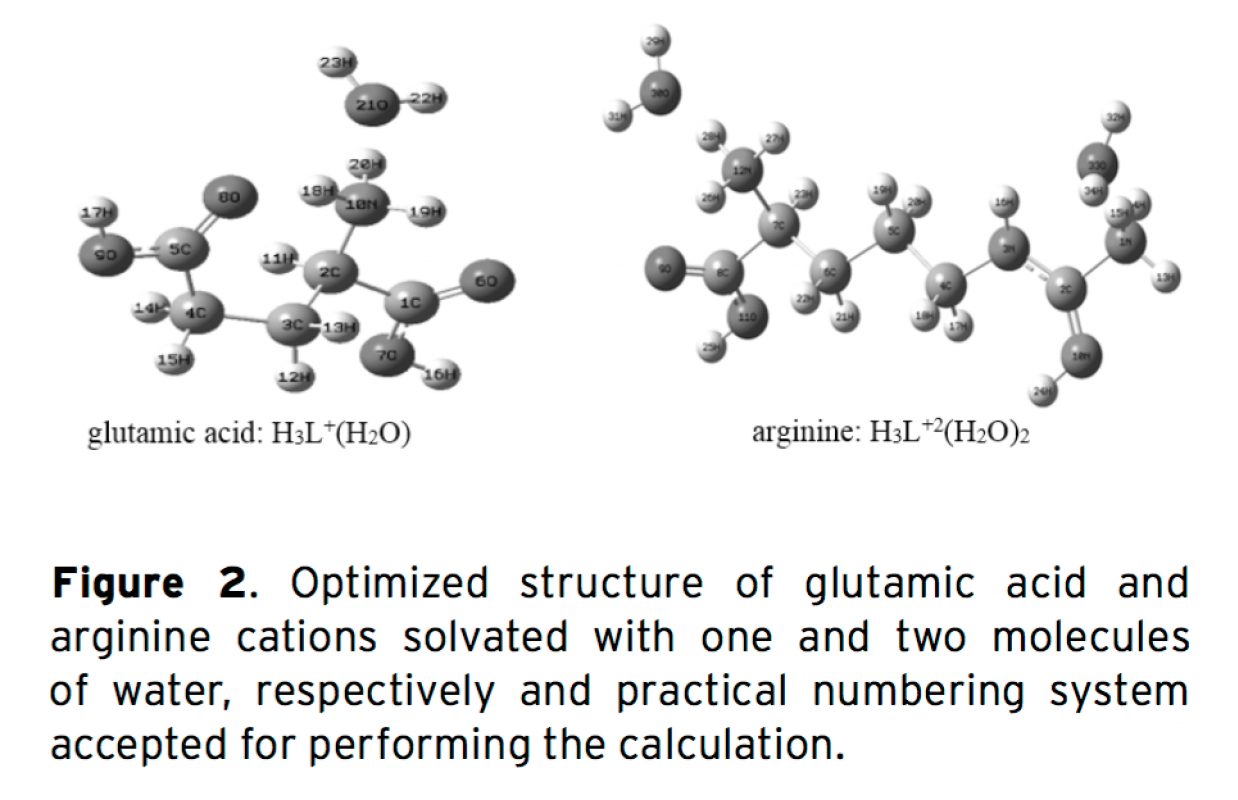
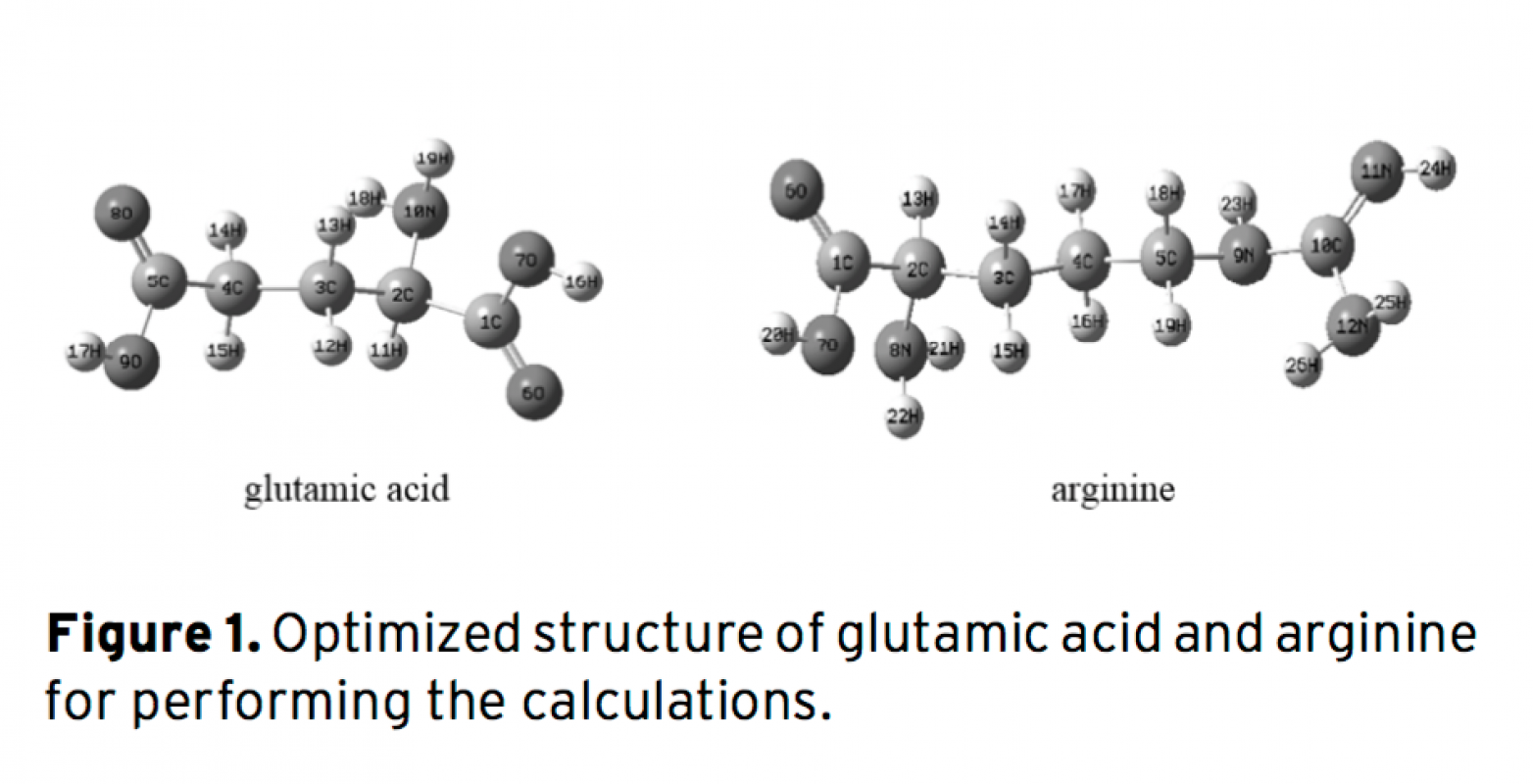
Gibbs free energy and acidic dissociation constants are two important thermodynamic properties of mo- lecules. A combination of ab initio with the density functional theory (DFT) and the polarized continuum model (PCM) of Tomasi’s method were utilized to calculate the acidic dissociation constants of arginine and glutamic acid in water. We applied the basis set at the B3LYP/6-31+G(d) level of theory for accurate theoretical predictions of pKa values. Furthermore, we have evaluated the molecular conformations and solute-solvent interactions of these molecules by the electronic structure theory (commonly DFT method). It was found that in alkaline aqueous solutions the cation, anion, and neutral species of arginine and glutamic acid are solvated with one, two, three, and four molecules of water, respectively. There are intermolecular hydrogen bonds bet- ween the existent species and water molecules. The atomic charges were investigated to analyze the reaction mechanism. In this study, it can be seen that there is a good correlation between experimental attained pKa values and the theoretical computed pKa values.
Gibbs serbest enerji ve asidik ayrışma sabiti, moleküllerin iki önemli termodinamik özellliğidir. Ab initio’nun yoğunluk fonksiyonel teori (DFT) ile kombinasyonu ve Tomasi’nin polarize olmuş sürekli dizi modelinden (PCM) yararlanılarak sudaki arjinin ve glutamik asitin asidik ayrışma sabitleri hesaplanmıştır. pKa değerinin teorik tahmini için B3LYP/6-31+G(d) teori seviyesi uygulaması kullanılmıştır. Ayrıca elektronik yapı teorisiyle (geleneksel DFT yöntemi) bu moleküllerin moleküler konformasyonları ve çözünen-çözücü etkileşimleri incelenmiştir. Elde edilen bulgulara gore alkali sulu çözeltilerde arjinin ve glutamik asitin katyon, anyon ve nötral türleri, sırasıyla, bir, iki, üç ve dört su molekülü ile çözünmüştür. Varolan türler ile su molekülleri arasında molekül içi hidrojen bağları vardır. Tepkime mekanizmasını analiz etmek için atomik değişimler araştırılmıştır. Bu çalışmada, deneysel pKa değerleri ile teorik pKa değerleri arasında iyi bir ilişki olduğu görülmektedir.
Download Article in PDF (534.7 kB)